Viewings: 5522
Some ancient philosophers believed that the air is the primary element or primary substance. They believed that he is not divided into simpler components. So, the ancient Greek philosopher Empedocles taught that the universe is composed of four elements - water, earth, fire and air.
In the XVII century, the English naturalist John Meow empirically came to the conclusion that one of the parts of air support combustion and life. He called "inflammable air".
Oxygen was allocated one hundred years after the opening of the m & e of CMV "fuel air". It was singled out simultaneously in England (Joseph Priestley) and in Germany (Carl Scheele) . Priestley was heated mercury in the air until, until it turned into a red powder. With further heating of the powder from his stood gas, which supported the burning better than normal air. The gas was oxygen.
Nitrogen was opened as follows. In 1752 Joseph black in experiments allocated from the air substance, which he called "the associated air". Twenty years later Daniel Rutherford in the study of properties of a gas formed after the combustion of charcoal, opened nitrogen (suffocating gas).
Neutral argon gas, which is 1% of the total volume of air that has been highlighted in 18 94, Argon allocated John Raleigh and Hives Ramsay. Then were assigned helium, neon, krypton, xenon and hydrogen.
Today it is established that air is composed of the following elements (numbers indicate the volume in%): Nitrogen (78,084); Oxygen (20,946); Argon (0,934); Carbon dioxide (0,033); Neon (0,000018); Helium (0,00000524); Methane (0,000002); krypton (0,00000114); Hydrogen (0,0000005); nitrogen Oxides (0,0000005); Xenon (0,000000087).
Air also contains some impurities that are in solid and liquid state. They all natural or artificial origin, have very different chemical composition, size, shape, and physical properties. These particles are called "aerosols". Particularly large amounts of aerosols industrial origin contained in the atmosphere of big cities. There one cubic centimeter contains thousands and even hundreds of thousands of particles. It is well known that over industrial cities in the atmosphere often "hanging" tens of thousands of tons of soot and dust.
Also aerosols in the atmosphere contains large particles of dust and water ice crystals. All these impurities play a very important role in atmospheric processes or in the formation of the weather. Water particles, for example, serve as the nuclei, which begins condensation of water vapor in the atmosphere. Therefore they are necessary for the formation of fog, clouds and eventually elements of precipitation (rain, snow it. D.).
The presence of aerosols in the atmosphere makes it less transparent, muddy. Through it harder passes solar radiation. Aerosols small size remain in the atmosphere for a very long time. During this time they are transported by air currents over great distances. In conditions of a stronger mixing of atmospheric aerosols climb to higher altitudes and fall, when the mixing process is slowed down. So at night, when the atmospheric gas is mixed less effective layer aerosols is lower. The process of distribution of aerosols in height and in the space of complex and depends on many factors.
The major constituents of the atmosphere, which admixtures with a low content, are sulphur dioxide (O) , nitrogen oxides, ammonia (N) , methane (CH) , carbon monoxide (CO) , ozone, and various organic compounds. Although these contaminants relative to the total weight of the air a bit, they are very substantially can affect conditions on the Ground. So, for example, increased content of carbon dioxide in the atmosphere from 0,029 in 1900 to 0,0334% in 1979 led to a marked increase in average air temperature in the surface layer. If the increase in carbon dioxide will continue, because of rising temperatures may create a critical situation due to ice melting in Greenland and Antarctica. The result is strongly increase the level of the World ocean and many coastal cities in the world will be under water.
Carbon dioxide absorbs and pereslushal-infrared radiation that is emitted by earth's surface. If it becomes larger, the Earth will continue to absorb the same amount of solar radiation, and to radiate into the environment will be less. So, its temperature will rise.
Dust and other particles that enter the atmosphere with volcanic eruptions and other pollution sources, can also affect surface temperature and surface air. The more you have, the more they delay solar radiation and thus lead to the decrease of the temperature of the planet.
There is an idea that is very useful "breathe ozone". So many will be surprised that ozone is a poison in that case, if it is contained in the air more than a certain (very small!) its part. Ozone is formed in the surface layer of air in the result of the activities of industry and transport. Nitrogen oxides and non-burnt hydrocarbons gases, interacting under the influence of solar radiation and create a thick haze (photochemical smog). In one cubic meter of smog contains up to 1 mg of ozone. This could dangerous. It affects vegetation, irritating to respiratory system and mucous membranes of eyes, has a negative impact on the earth's flora and fauna. Unfortunately, currently "ozone smog" observed in many large cities of the world.
In nature is infinitely repeating the cycle of substances. Participate and components of the air - nitrogen, oxygen and carbon dioxide. When nitrogen is in the gaseous state, it is a MIME inert gas. But in the compounds, which are called nitrates, it plays an important role in metabolism in the animal and plant world. Nitrates are created by plants, bacteria which capture free nitrogen from the air. Animals eating plants consume nitrates. Green plants remove carbon dioxide from the air and with the help of photosynthesis free oxygen. Estimates show that all vegetation of earth uses for the year of about 550 billion tonnes of carbon dioxide. At the same time they release oxygen in the amount of about 400 billion tons of Carbon dioxide being released into the atmosphere when the plants burn or rot when I breathe people and animals, when evaporate mineral springs and volcanoes erupt. The duration of a full cycle for each gas is different. So, carbon dioxide takes an average of one to three years, the oxygen - three thousand years, and nitrogen - hundred million years.
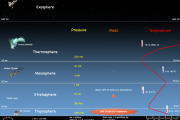
With a thermometer in a balloonThe higher the mountain we go, the colder it becomes. If we will rise by plane at a height of 9 km and there (overboard) temperature will drop to minus 40-50°North as above? How long the temperature will decrease as we rise up? It is clear that for a further rise to us having to move out of the plane into a rocket. But we must not forget to take a thermometer to measure the temperature of the air. We will make our ascent rocket in the middle lane in the summer when the air temperature on Earth is +2 7 degrees C. We have chosen such a temperature of not only because it real in these conditions, but also because it meets all the number of degrees Kelvin, namely 300 OC K. It is not important, just more convenient. As we rise on every kilometer of the temperature decreases by 6.5 degrees. Suddenly, at the height of about 12-13 km temperature ceases to decrease. We reached the bottom of the ozone layer, the storeroom heat in the atmosphere. Here is autonomouse. This region of the atmosphere in which temperature decreases with height, was called the troposphere. The word "tropo" means the variability. This applies to the temperature .
Altitude the temperature variations of the atmosphere were studied long before the invention of missiles and aircraft. Study of the temperature of the atmosphere began in the middle of the XVIII century For this raised the thermometers in air snakes. In the end of XVIII century thermometers were raised by balloons. And it was very effectively. So, a well-known physicist and chemist Joseph Gay-Lussac in 1804 he made two ascent in a balloon. In the second rise, he reached a height of 7 km of These rises were very informative. The scientist is not only measured the temperature of the air at different heights, but its moisture content, and took air samples at different levels. The analysis of these samples of air and made it possible to conclude that at these heights the composition of the air remains constant. With increasing altitude decreases only its density.
In the same 1804 balloon flight made Russian academician I. D. Zakharov.
Later, these investigations were carried out regularly. Especially mass they began in the second half of the XIX century was reached a record height of 11.2 km did English meteorologist James Glaisher. Balloon with the aim of atmospheric research in 1887 climbed the great Russian chemist D. I. Mendeleev. Thus, using balloons managed "test" the entire troposphere.
Above 11 km began to lift on high altitude balloons devices that can measure the temperature (and other parameters) atmospheric gas without human intervention. Such a device was invented in 1892, Hermite and J. Besancon and was named meteogram. It is with the help of metagraf in 1928 and was discovered HP Outboard, higher than 12 km, the temperature is not reduced. In this result no one wanted to believe - too paradoxical it seemed. So I decided that measurements are wrong. But when the same result showed matography in hundreds of flights of high-altitude balloons, had nowhere to go, he believed. Had to acknowledge the presence in the atmosphere above the troposphere layer, where the altitude profile of the temperature feels handling inversion. Therefore he was named layer inversion.
In the first measurements using Meteograms it was found that the troposphere at different latitudes has a different length, height (from 8 to 12 km).
We will continue to climb up on. From 12 to 20 km, the temperature does not practically change with height. It is said that this isothermal layer, that is, the layer with constant temperature ("from" means "equal", "identical"). From 20 to cm temperature with increasing altitude increases. If in the troposphere temperature difference in height was positive, at these altitudes it is negative. Above 47 miles (51 km) temperature again remains unchanged. This is the second isothermal layer. The whole area from 12 to cm called the stratosphere ("STRATO" - "layered"). Stratosphere at the upper ends with stratopause. The temperature on STRATO-pause reaches approximately 10-20°N
If tropospheric air to add the one in the stratosphere, you will get 99% of all air. Above km is only about 1% of all air.
Above stratopause is another (intermediate) sphere. She is called the mesosphere ("mezos meaning"- "intermediate") . Here again, the temperature decreases with altitude (as in the troposphere). The mesosphere extends to a height of 8 6 km. At the top of metope-ry (the mesopause), the temperature is decreased to minus 75-90°N
On the mesopause altitude temperature profile again breaks. Above mesopause temperature increases with height (as in the stratosphere) . This part of the atmosphere is called the thermosphere ("thermo" - "heat") . In the thermosphere the temperature reaches many hundreds of degrees.
Does this mean that once there, we with our rocket will go to hell? Far from it! Here is so deep vacuum that the concept of the temperature has the meaning different from that adopted by us in daily life. While in normal conditions (on the earth) , we temperature measure the degree of Hotness of the body. In the case of gas this means that the higher the temperature the gas, the more the speed of its molecules. In other words, the faster moving particles of the gas, the more the temperature. Talking about the temperature of one particle is impossible. You can only talk about the temperature of the entire gas. Gas particles must face and share with one another energy (as billiard balls). The lower the density of the gas, the less often collide the particles of which it consists. At sea level air molecules collide with each other so often that between collisions molecule flying just a few millionths of a centimeter. This path is called the free path of a particle. At an altitude of 100 km length free path of particles reaches one meter, and in the thermosphere at an altitude of 300 km - 10 km So the thermosphere we should talk not just about temperature and kinetic temperature of the particles. It is measured by the kinetic energy of the particles, their speed. The kinetic energy of particles in the thermosphere are very high, so high, their kinetic temperature. But this high temperature we, once there, would be unable to feel, because the density of the gas is negligible. Moreover, the part of our body, which would not fall the sun's rays, would be ice cold (although there kinetic temperature reaches many hundreds of degrees).
Above thermosphere, there is another sphere - the exosphere ("skso" - external"). This region of the atmosphere so named because here the particles can have speeds that are more than the first cosmic speed (11.2 km/s) . At such speeds particles overcome the force of gravity and fly away beyond the earth's atmosphere.
















